iosBio signs exclusive worldwide licensing agreement granting ImmunityBio rights to OraPro™ oral vaccine platform technology for COVID-19
- iosBio licenses its OraProTM thermal stabilization technology to ImmunityBio for oral delivery of ImmunityBio’s second generation hAd5 vaccine candidate to protect against COVID-19
- ImmunityBio’s oral T cell vaccine candidate could potentially address mutations in the spike (S) protein
Burgess Hill, UK, January 12, 2021—iosBio (‘the Company’),a UK-based biotechnology company developing next-generation vaccines that can be administered orally, today announced an exclusive worldwide licensing agreement with ImmunityBio, Inc. a US-based, privately-held clinical-stage immunotherapy company, for iosBio’s OraPro™ vaccine platform technology currently being investigated in trials of ImmunityBio’s second-generation human Adeno (hAd5) COVID-19 vaccine candidate.
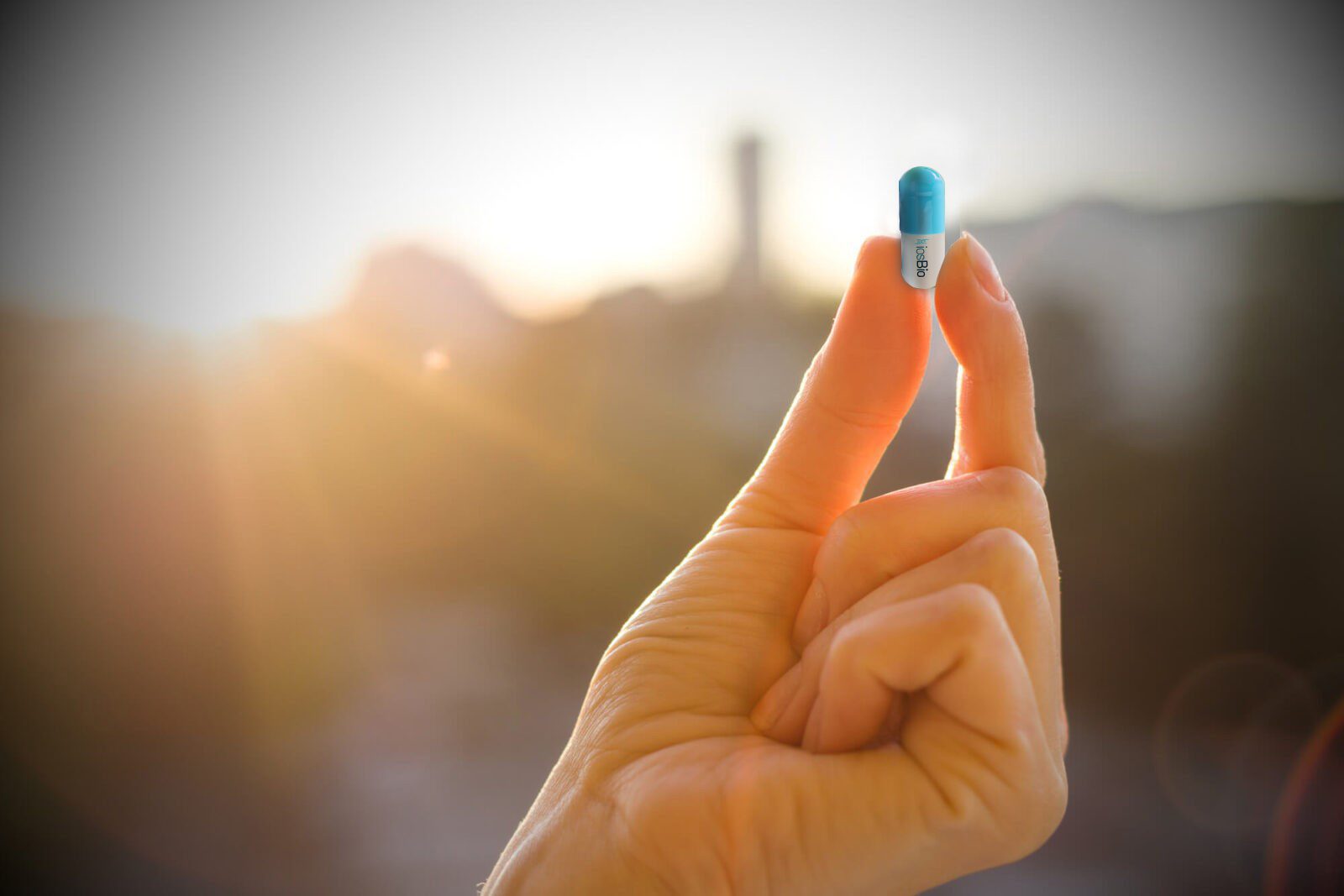
OraPro™ is iosBio’s patented oral delivery vaccine platform technology that enables oral administration of thermally-stable, viral vector vaccines. These vaccine vectors are engineered to withstand temperatures of up to 50°C, allowing them to pass through the hostile conditions in the stomach without loss of efficacy and providing long-term product stability at ambient temperatures. Oral administration delivers the vaccine directly to mucosal associated lymphoid tissue (MALT), generating mucosal, systemic and T-cell immune responses.
Under the terms of the licensing agreement, iosBio has granted ImmunityBio exclusive rights to utilize its OraPro™ platform technology for the oral delivery of ImmunityBio’s human Adeno (hAd5) COVID-19 vaccine candidate.
iosBio will be eligible to receive royalties on net worldwide sales of the approved oral vaccine.
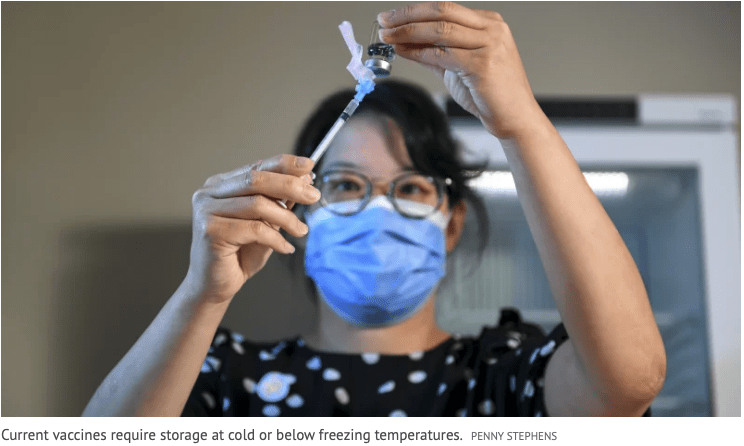
In a BARDA-sponsored study of vaccinated non-human primates, subcutaneous and room temperature oral formulations of ImmunityBio’s vaccine candidate inhibited SARS-CoV-2 virus replication to undetectable levels and cleared infection within days in 100% of vaccinated animals (10 of 10). The vaccine candidate, which delivers both outer spike (S) and inner nucleocapsid (N) antigens, induced T cells and antibodies leading to reduction of SARS-CoV-2 viruses in both lungs and nasal passages within seven days. The oral capsule formulation of the vaccine candidate was effective at room temperature in the non-human primate study suggesting that it may not require the cold chain logistics that can impede global distribution.
Pending ImmunityBio’s discussions with the US Food and Drug Administration (FDA), the oral vaccine will enter Phase I trials as a prime and boost, and will be explored to provide a boost to subcutaneous vaccinations. Twenty participants have already completed testing in ImmunityBio’s Phase I trial at Hoag Hospital Newport Beach, California, which evaluated both low and high doses of subcutaneous hAd5, with zero grade 3/4 adverse events reported.
Chairman of iosBio, Wayne Channon, said: “We are delighted to be able to support ImmunityBio with the oral delivery of its second-generation COVID-19 vaccine candidate, through the licensing of our OraPro thermal stabilization technology. This technology is key to developing vaccines that can be administered orally without loss of efficacy and has the potential to truly transform vaccine development.
“Oral vaccines have the potential to overcome global challenges of traditional vaccines, many of which need to be stored and transported at freezing temperatures. Oral vaccines are more cost effective to produce and can be easily stored and transported around the world. They also have the potential to be self-administered, reducing health systems’ dependency on trained health professionals to run immunisation programmes and present a future where people could have vaccines delivered straight to their door.”
Patrick Soon-Shiong, M.D., Chairman and CEO of ImmunityBio, said: “As we see multiple mutations in the SARS-CoV-2 spike protein, there is an urgent need for a vaccine that not only offers immediate protection but also activates T cells to clear the virus. When multiple mutations occur at the receptor-binding domain of the spike protein, it renders antibodies ineffective. Our next-generation vaccine design drives both antibody and T cells to S and N protein, and so could potentially serve as a universal boost to current vaccines that focus only on the monovalent S protein, as well as address future mutations of the S protein.
“It is also critical to develop a room-temperature, stable oral formulation of the vaccine, one that will provide mucosal immunity. We’re doing that with iosBio’s unique oral vaccine delivery platform technology that has the potential to transform the way people take vaccines and addresses the challenges of storage and global distribution of vaccines. In our BARDA-sponsored, non-human primate challenge study, administering the oral formulation as a boost achieved 100 percent viral protection against a challenge and induced both memory B cells and Th1-dominant T cells, which cleared the virus. We are now moving urgently through clinical development in the US and globally.”
Ends
For further information please contact:
Consilium Strategic Communications
Mary-Jane Elliott, Melissa Gardiner, David Daley,
IosBio@consilium-comms.com
Tel: +44 (0) 20 3709 5700
NOTES FOR EDITORS
About iosBio
iosBio is a UK based biotechnology company developing next generation vaccines that can be administered orally.
The Company’s proprietary OraPro™ thermal stabilization technology enables the oral administration of thermally stable, non-replicating viral vectors that can be delivered sublingually via the gastrointestinal (GI) tract and other routes. These vaccine vectors are engineered to withstand temperatures of up to 50 degrees, allowing them to pass through the hostile conditions in the stomach without loss of efficacy and providing long term product stability at ambient temperatures.
iosBio is developing vaccines designed to stimulate mucosal, systemic and T cell immune responses, providing robust immunity to a number of infectious diseases including COVID-19, Zika and influenza.
iosBio is headquartered in Burgess Hill, UK. For more information visit: iosbio.com
About ImmunityBio
ImmunityBio, Inc. is a late-clinical-stage immunotherapy company developing next-generation therapies that drive immunogenic mechanisms for defeating cancers and infectious diseases. The company’s immunotherapy platform activates both the innate (natural killer cell and macrophage) and adaptive (T cell) immune systems to create long-term “immunological memory.” This novel approach is designed to eliminate the need for high-dose chemotherapy, improve upon the outcomes of current CAR T-cell therapies, and extend beyond checkpoint inhibitors.
ImmunityBio’s platform is based on the foundation of three separate modalities: antibody cytokine fusion proteins, synthetic immunomodulators, and second-generation human adenovirus (hAd5) vaccine technologies.
Anktiva™ (ImmunityBio’s lead cytokine infusion protein) is a novel interleukin-15 (IL-15) superagonist complex and has received Breakthrough Therapy and Fast Track Designations from the U.S. Food and Drug Administration (FDA) for BCG-unresponsive CIS non-muscle invasive bladder cancer (NMIBC). The company is also in Phase 2 or 3 trials for indications such as first- and second-line lung cancer, triple-negative breast cancer, metastatic pancreatic cancer, recurrent glioblastoma, and soft tissue sarcoma in combination with the company’s synthetic immune modulator (aldoxorubicin).
ImmunityBio is also developing therapies, including vaccines, for the prevention and treatment of HIV, influenza, and the coronavirus SARS-CoV-2 with its second-generation human adenovirus (hAd5) vaccine technologies.
NantKwest Transaction
As previously announced, on December 21, 2020, ImmunityBio entered into an agreement to combine in a stock-for-stock transaction with NantKwest, Inc. (NASDAQ: NK). The combination, which is expected to close in the first half of 2021, would create a leading immunotherapy and cell therapy company focused on oncology and infectious disease.